The majority of the WI hazardous waste administrative codes reflect mandates in the federal RCRA regulations. Ultimately, they streamline the management of hazardous waste pharmaceuticals. And, even more important, protect human health and the environment.
Over the next three blog posts, we’ll address three major initiatives that WI healthcare facilities must now follow:
- Subchapter P – Hazardous Waste Pharmaceuticals Rule
- Definition of Solid Waste Rule
- Generator Improvement Rule
This post addresses the first initiative. We’ll cover the next two in upcoming blogs.
WI New Hazardous Waste Pharmaceuticals Rule – 666.500-666.510
Below are eight main points of the new WI Hazardous Waste Pharmaceuticals Rules. They mirror the EPA’s 266 Subpart P Regulations. Read on, or click to the section you’d like to read more about.
- All Healthcare Facilities Must Comply
- Know what Hazardous Waste is on Site
- Only Give Reverse Distributors Potentially Credible Hazardous Pharmaceutical Waste
- Properly Label Containers and Use Correct Manifest Code When Shipping
- Don’t Flush Hazardous Pharmaceuticals Down the Drain (aka Sewer-Ban)
- These Five Hazardous Pharmaceutical Wastes are also DEA Controlled Substances
- Liquid Nicotine is Acute Hazardous but OTC Nicotine Replacements Are Not
- “RCRA empty” Containers No Longer Need to be Triple Rinsed Before Disposal
Also, if you are a Small or Large Quantity Generator, don’t forget to add this to your “to do” list if you have yet to do so:
Here’s a breakdown of each point:
1. All Healthcare Facilities Must Comply – 666.500 (3)
Basically, if your facility provides healthcare to humans or animals, it must follow many, if not all, of the new hazardous laws, regardless of your size.
Your facility must follow these new rules if it:
- Provides preventative, diagnostic, therapeutic, rehabilitative, maintenance or palliative care
- Offers counseling services, assessments or procedures, with respect to patients’ physical, mental condition, or functional status
- Distributes, sells, or dispenses pharmaceuticals, including over-the-counter pharmaceuticals, dietary supplements, homeopathic drugs, or prescription pharmaceuticals.
This pretty much covers all:
- Hospitals
- Health Clinics
- Psychiatric and Substance Abuse Centers
- Ambulatory Services and Ambulatory Surgical Centers
- Physicians’ Offices
- Optical and Dental Providers
- Chiropractors
- Veterinary Clinics and Hospitals
- Long-Term Care Facilities (administer pharmaceuticals to one or more individuals there)
- Pharmacies (including Mail-Order and Long-Term Care)
- Retailers of Pharmaceuticals
- Wholesale Distributors
- Third Party Logistics Providers that serve as forward distributors
In a nutshell, the new rules will affect these three types of healthcare facilities that regularly accrue hazardous waste pharmaceuticals.
Namely:
- Large Quantity Generators (LQG): Generate more than 2,200 lbs. of hazardous waste per month.
- Small Quantity Generators (SQG): Generate more than 220 lbs. of hazardous waste, but less than 2,200 per month.
- Reverse Distributors: Those who receive or take back prescription pharmaceuticals from another organization to verify a manufacturer’s credit.
Very Small Quantity Generators (VSQGs), or those who generate less than 220 lbs. of hazardous waste pharmaceuticals per month, are not beholden to Subchapter P, except for the Sewer Ban and Nicotine Amendment. They can continue to follow their previous way of handling their pharmaceuticals and acutely hazardous P waste under NR 662. However, they may opt into it. Or, they can also send their hazardous waste pharmaceuticals off to an affiliate site that operates under Part 266 Subpart P.
2. Know what Hazardous Waste Is On Site –– 666.501
A review of the formulary list from the pharmacy will help you determine what is a hazardous pharmaceutical waste — and what isn’t. Your pharmacist will know – and may likely even tell you that a majority of what’s on the shelf is actually non-hazardous. In fact, only about 8% of pharmaceuticals are hazardous, but the tricky part is knowing which ones are.
The whole point of Subchapter P is to make sure you properly dispose of pharmaceutical hazardous waste. Most importantly, not flush it down the sewer, where it can pollute the waterways.
Commingling of hazardous and non-hazardous waste pharmaceuticals in the same container is acceptable. But remember then, the whole container is now hazardous waste.
3. Only Give Reverse Distributors Potentially Credible Hazardous Pharmaceutical Waste – 666.504
In years past, healthcare facilities typically gave most or all of their pharmaceutical waste to Reverse Distributors. This was regardless of whether they thought they could get credit for their expired medication. Subchapter P changes things.
The EPA now says healthcare facilities can only give Reverse Distributors “potentially creditable” pharmaceutical waste. Furthermore, pharmaceutical waste must be identified on site as potentially creditable or non-creditable pharmaceutical waste, and not hauled off site and identified elsewhere. Finally, it must be evaluated within 30 days to determine its creditability.
Below are a couple lists to help you determine what waste is potentially creditable.
Potentially Creditable Waste:
- Drugs in the original manufacturer packaging (except recalls)
- Pharmaceutical waste that has not been dispensed
- Unexpired waste or less than 1 year past expiration date
Non-Creditable Waste:
- Broken or leaking container
- Repackaged
- Dispensed
- Expired (greater than 1 year past expiration date)
- Investigational new drugs
- Contaminated PPE
- Floor Sweepings
- Clean-up Material
Evaluated (“Creditable”) Waste:
- Manufacturer credit already determined/verified
- Pharmaceuticals destined for a treatment, storage and disposal facility (TSDF)
Non-creditable waste will no longer be able to flow through a Reverse Distributor. It must be disposed of by a permitted waste facility. Remember, once opting into Subpart P, all non-credible hazardous pharmaceutical waste must be disposed of within the year of when the first item was placed inside of the container. Some of the ways to keep track of this date is to either mark the date on the container when the first item was placed inside of it, or maintain an inventory log.
Many healthcare facilities used to transfer hazardous pharmaceuticals that were stored in satellite areas into a larger drum in a main accumulation area. With Subpart P, satellite accumulation areas are no longer applicable. This means that when transferring non-credible hazardous waste pharmaceuticals between containers, say from a smaller area to a larger one, the earliest (i.e., oldest) date must be placed on the receiving container.
The flow chart below can help your healthcare facility determine the proper disposal method for its pharmaceutical waste.
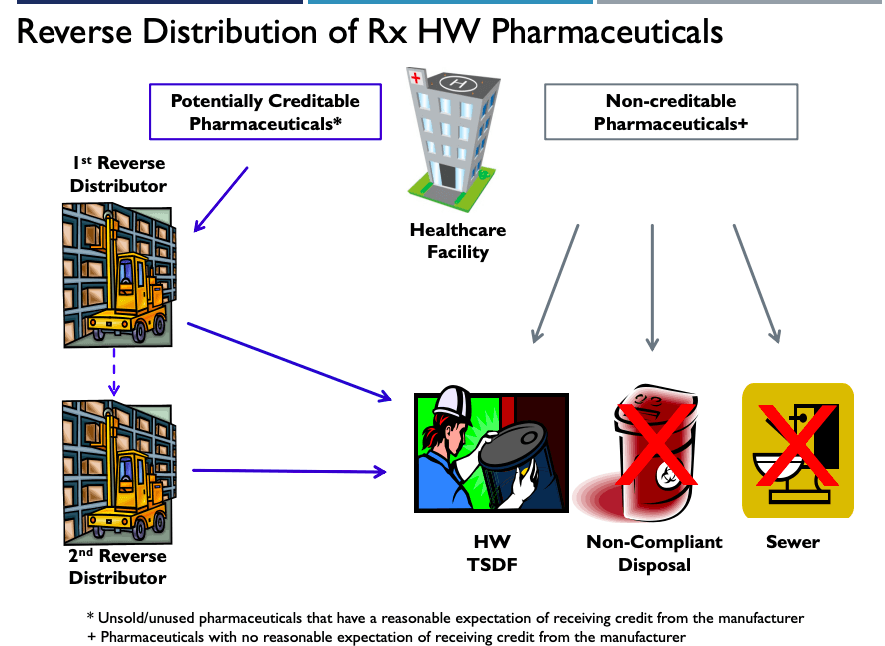
4. Properly Label Containers and Use Correct Manifest Code When Shipping – 666.502
The following matrix will help both healthcare facilities and reverse distributors. It summarizes the timing for on-site accumulation and shipping standards for potential creditable pharmaceuticals.
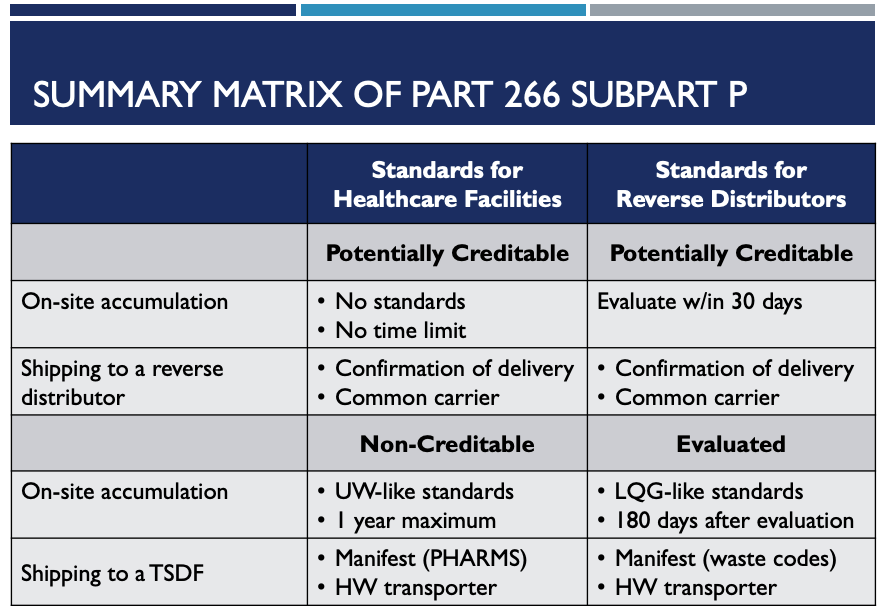
For evaluated and potentially creditable hazardous waste pharmaceuticals, the reverse distributor is exempt from RCRA Rules. Follow your reverse distributor’s shipping requirements.
Healthcare facilities need to have a manifest when shipping non-creditable hazardous waste pharmaceuticals. The good news is, this process will become a little easier. Rather than having to write various hazardous waste codes on manifests, you’ll only need the code ““PHRM”. Remember though, that if the receiving state’s treatment facility has not adopted the rule, waste codes will still be required on the manifest.
You must use DOT-rated containers for hazardous waste or any DOT hazardous material. In addition, you’ll need to follow your facility’s internal procedures for collection containers and DOT Regulations when shipping the waste. Make sure your containers have their proper DOT hazardous waste labels on them before shipping.
They must have a “Hazardous Waste Pharmaceuticals” label if that is what is inside of them, along with a label noting if it is any one of these:
- Toxic
- Flammable
- Corrosive
- Reactive
All containers must be structurally sound, with the lid closed, and include a date of when the first item was put into the container. Keep in mind, the disposal must take place within one year of that date.
Finally, you must maintain an inventory or log of your waste and keep records of your manifests that show you are keeping track of what has been disposed. You should receive your signed manifests from the operators of the hazardous waste treatment facility within 30 days. If you have not received anything within 35 days, reach out to them. LQGs have 45 days, and SQGs have 60 days to report to the DNR if they have not received a manifest. You’ll need to note your followup with the transporter when sending along with a copy of the original manifest, to the DNR.
5. Don’t Flush Hazardous Pharmaceuticals Down the Drain (aka Sewer-Ban) – 666.505
To help get pharmaceuticals out of our national waterways, one major regulation you are likely already following is the nationwide EPA Sewer Ban. Wisconsin adopted this EPA law in August, 2020.
While the sewer ban specifically addresses not flushing hazardous waste pharmaceuticals down the drain, it is worth noting that the WI DNR stresses it is a best practice to not sewer any hazardous material.
Flushing hazardous pharmaceutical waste will be met with harsh penalties. So, if your facility disposes of hazardous waste pharmaceuticals by sending them down the sink or in the toilet, don’t do it now.
If you’re unsure of where to start, or wonder what’s a hazardous waste, it’s best to obtain a formulary list. You can get this from your pharmacist or pharmaceutical provider to make you aware of what hazardous pharmaceutical waste you have on site.
6. These Five Hazardous Pharmaceutical Wastes are also DEA Controlled Substances –666.505
Here are five DEA controlled substances that are also RCRA Hazardous Waste Pharmaceuticals. You can’t sewer these:
- Chloral/Chloral Hydrate (Acetaldehyde, Trichloro, Aquachloral, Noctec, Somnote, Supprettes)
- Fentanyl sublingual spray (Subsys)
- Phenobarbital (Bellergal-S Donnatal Luminal)
- Testosterone gels/solutions (Androgel Axiron Foresta, Testim)
- Valium gel/injectable (Diazepam, Diastat)
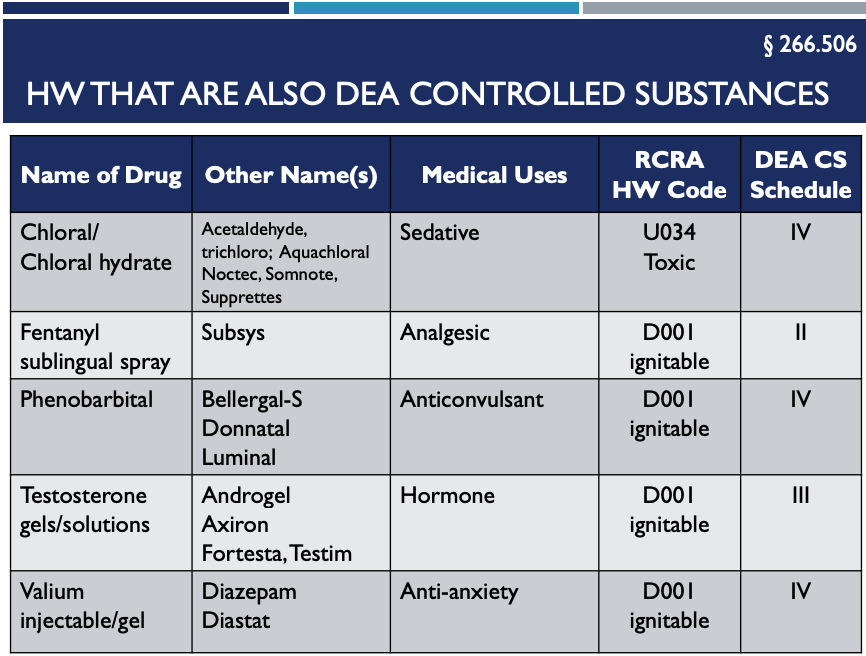
Note that the above five DEA hazardous substances are EXEMPT from RCRA if they are:
- Not flushed down the drain,
- Managed in compliance with DEA Regulations,
- Destroyed by a method that the DEA has deemed in writing to meet their non-retrievable standards, or
- Thermally destroyed at a permitted hazardous waste disposal facility.
7. Liquid Nicotine is Acute Hazardous but OTC Nicotine Replacements Are Not –666.500(9)
All generators are subject to follow two new provisions of the Nicotine Amendment. First, the Federal drug administration over-the-counter nicotine replacement therapies are no longer acute hazardous waste. They are no longer classified as P075. That is because these over-the-counter formulations are known to have lower concentration levels of nicotine.
However, prescription nicotine therapies and nicotine vaping fluids continue to be P-listed hazardous waste when disposed. Prescription nicotine products contain higher levels of nicotine. Vaping fluids also contain higher levels, which is why these are still on the P listing.
8. “RCRA empty” Containers No Longer Need to be Triple Rinsed Before Disposal – 666.507
The new 266 Subpart P Regulations cover other areas, including defining “RCRA Empty”. These as shown in the chart below.
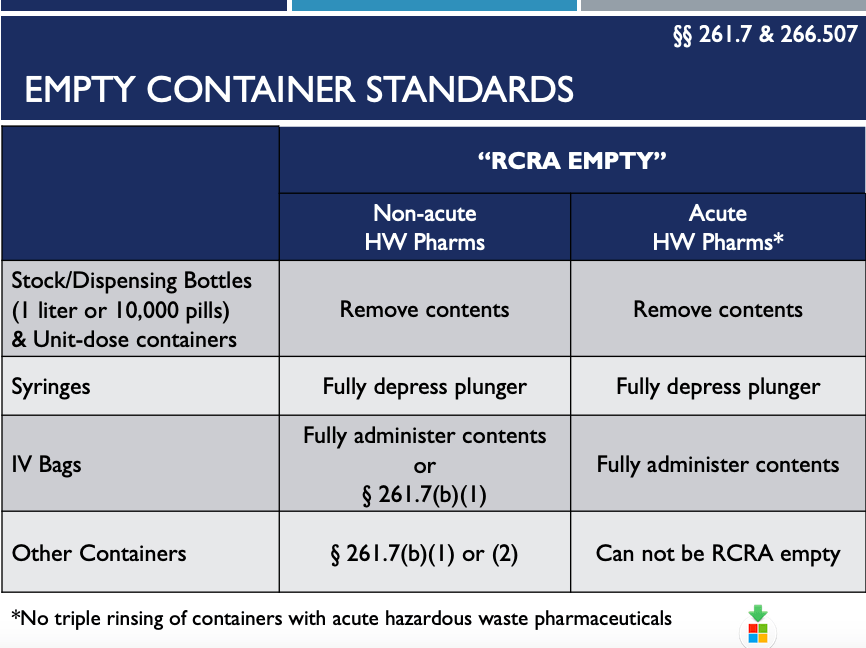
As you can see, “RCRA Empty” means to fully remove medicine from dispensing bottles. For syringes, fully depress the plunger. And, for IV bags, fully administer contents before disposing of it. Basically, once you’ve removed everything from it by pouring, pumping, and aspirating the medicine, it is “RCRA Empty”.
Residues remaining inside of a container will no longer be regulated as hazardous waste pharmaceuticals.
What does this “RCRA EMPTY” change mean for you?
In the past, many healthcare facilities triple rinsed containers when there was still a trace amount of hazardous waste pharmaceuticals in them. No more. Triple rinsing of RCRA pharmaceutical empty containers with acute hazardous pharmaceutical waste is no longer required or allowed.
Notify the EPA that you’ll be operating under Subchapter P
To let the EPA know you will be operating under Subchapter P, it is requiring the submission of a one-time notification using this site EPA ID form: 8700-12.
LQG’s/SQG’s
Large and Small Quantity Generators must submit this form during normal biennial reporting cycle. This means that, for those who normally report 2020 annual waste numbers, your deadline to submit this form is March 1, 2021. Because the law is new, the DNR will work with you to make sure your facility is in compliance.
Healthcare facilities are not required to fill out box 10.B “Waste codes for federally regulated hazardous wastes” with respects to hazardous waste pharmaceuticals. You do need to fill out this box for other hazardous waste generated onsite.
Keep a copy of this notification on file for as long as you are subject to this subchapter.
VSQG’s
Very Small Quantity Generators must complete form 8700-12 only if they decide to opt into Subchapter P. There is no timeline for submittal. If a Very Small Quantity Generators is part of a larger company, it may consolidate its waste within its larger company that is already operating as a large quantity generator. If not, they can continue to follow their previous way of handling their pharmaceuticals and acutely hazardous P waste under NR 662.
While it’s a lot to take in, these changes clarify hazardous waste pharmaceutical disposal for healthcare facilities. And, help them be a better friend to the environment.
As always, if you have any questions, give us a call at 608-257-7652 or contact us at meriinc.com.
###



